Destroying Cancer, Differently
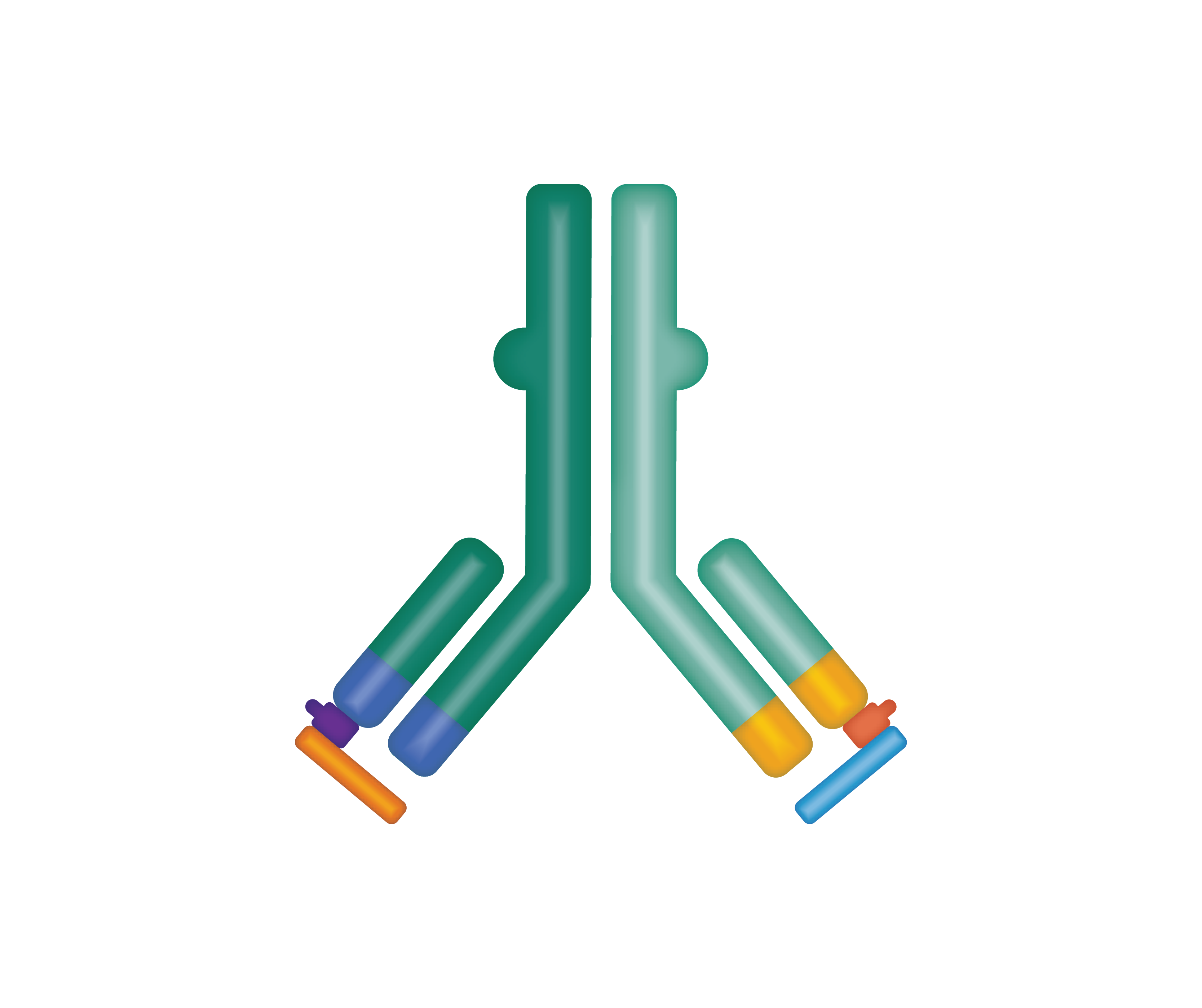
Probody® therapeutics are designed to outsmart cancer by exploiting the unique conditions of the tumor microenvironment by localizing treatment in the tumor and limiting activity in healthy tissue. We design Probody therapeutics to mask its target binding region. This mask reduces activity and toxicity in healthy tissue. Our novel therapeutics take advantage of the high levels of protease activity in the tumor microenvironment. Their target-binding regions are masked to limit activity —and toxicity— in healthy tissue. When Probody therapeutics encounter active proteases near tumor tissue, the mask is designed to be removed so that the unmasked therapeutic can bind to the tumor target.
We believe our Probody therapeutics have the potential to:
- Create or widen therapeutic window – the balance between a therapy’s dose that is effective without causing unacceptable toxicity
- Enable new combinations of drugs previously not possible due to toxicities
- Expand the universe of viable therapeutic targets by opening “undruggable” target space
- Make new treatment options possible for more patients
Designed to selectively activate in the presence of cancer
Probody® therapeutics have been intentionally designed to bind selectively to tumors, and not to healthy tissue, with the goal of minimizing toxicity and creating safer, more effective cancer therapies. This revolutionary approach and our Probody drug candidates are designed based on a deep understanding of tumor protease biology.
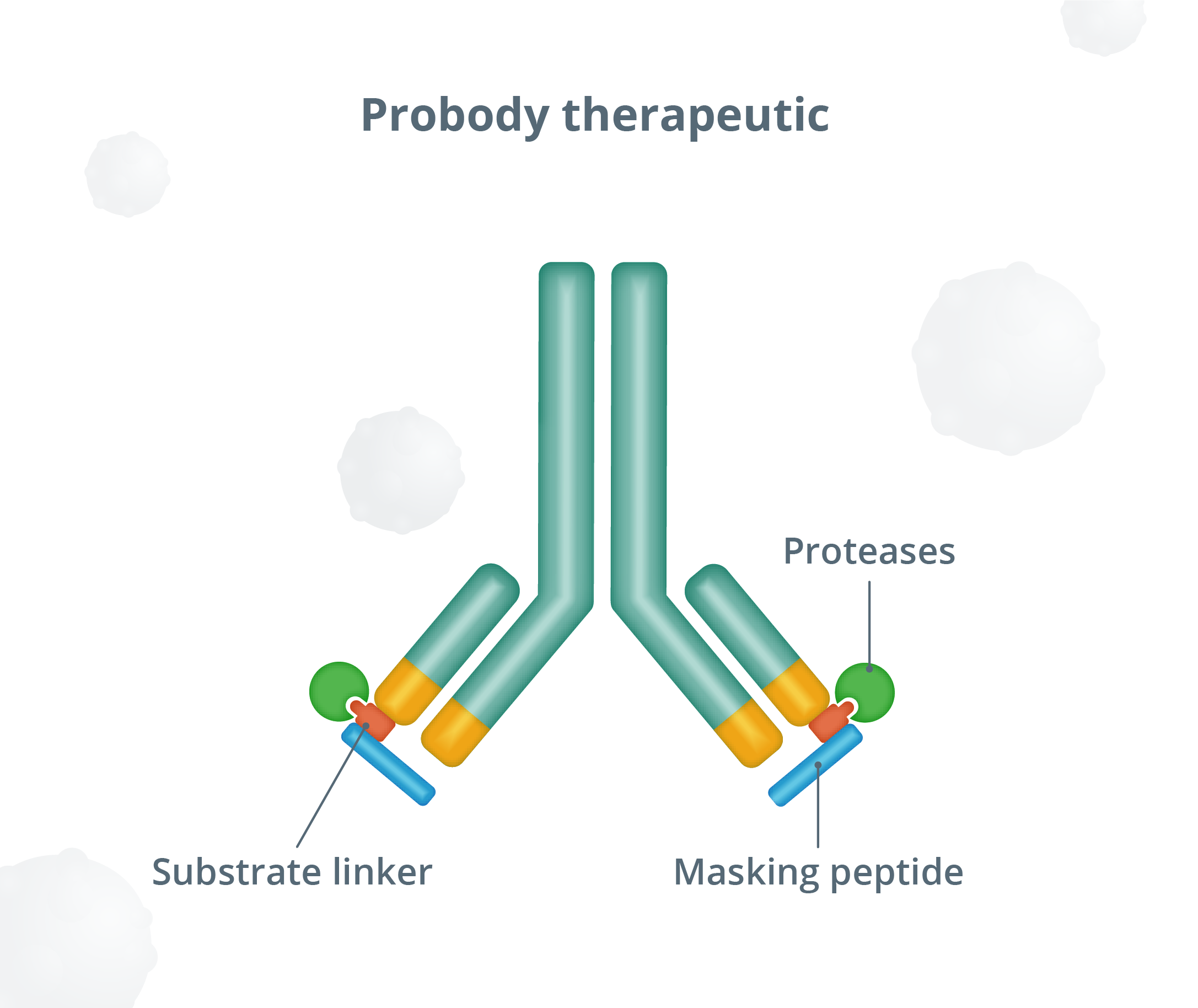
A pioneering antibody prodrug technology.
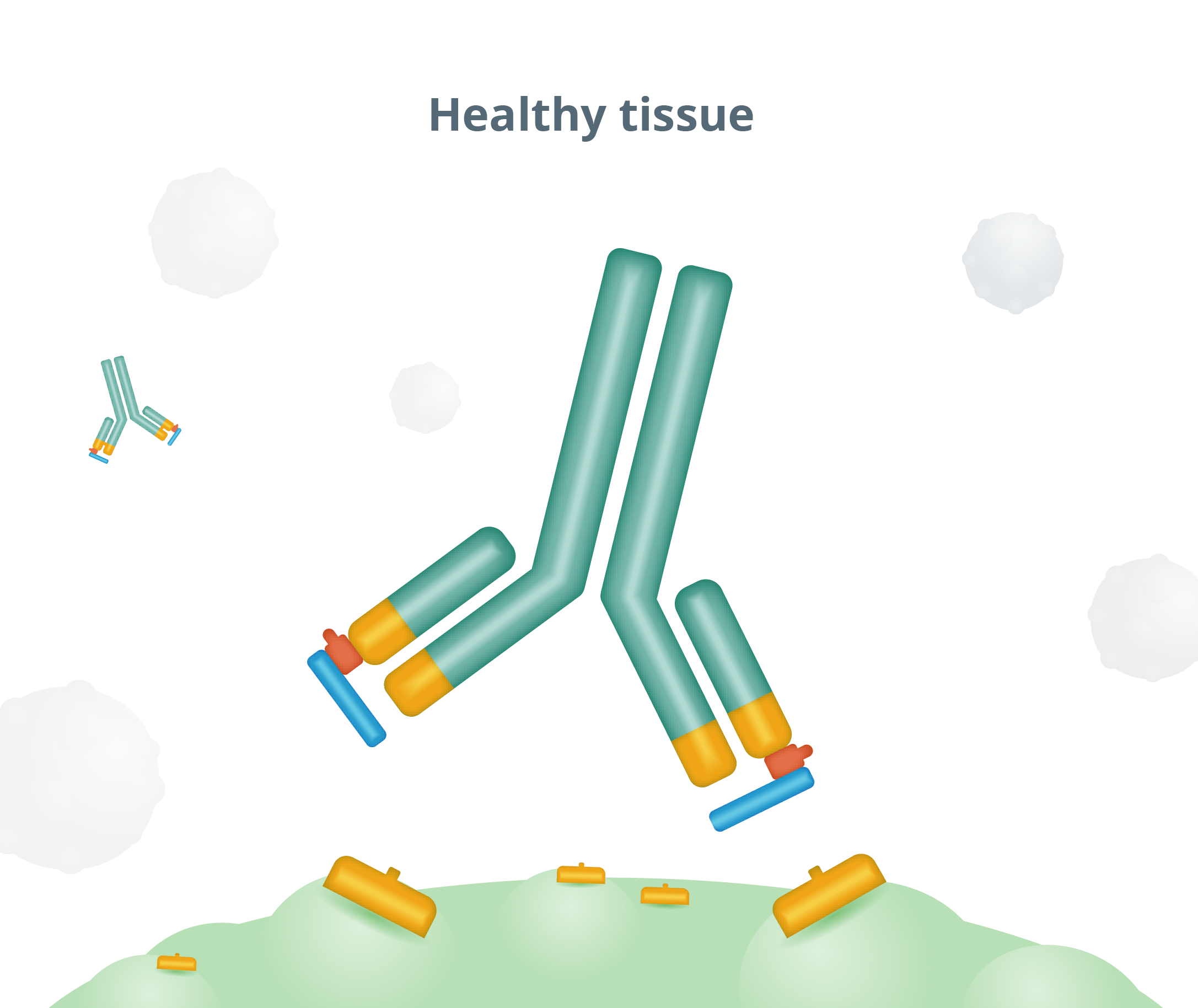
The “masking” peptide is designed to limit the ability of Probody therapeutics to bind to healthy tissue—thereby helping to minimize toxicities.
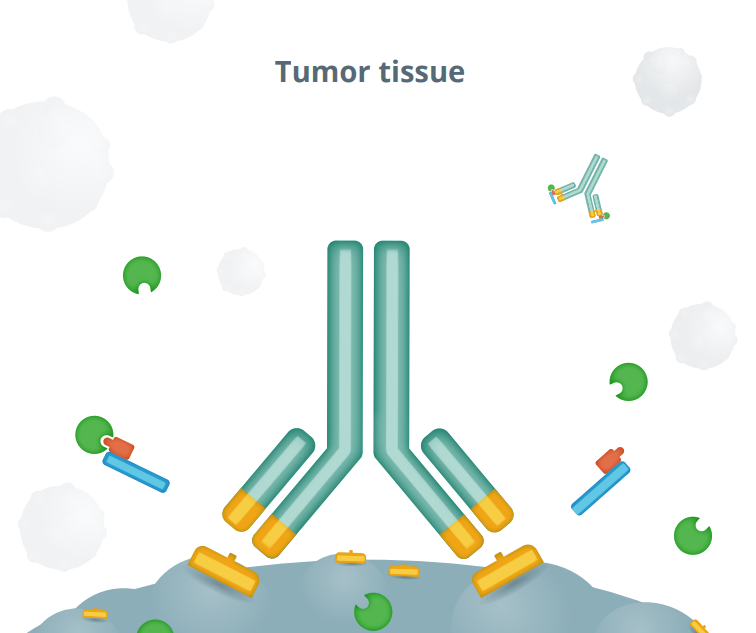
In the tumor environment, protease enzymes are expected to cleave the substrate, removing the “mask” and activating the Probody therapeutic to bind to its target on cancer cells.
Next generation of targeted antibody therapeutics.
A versatile platform
Our highly versatile Probody® platform has potential utility across the most compelling antibody therapeutic modalities: Antibody drug conjugates, T-cell-engaging bispecifics, and cytokine therapy. We are tackling big challenges with these modalities:
- Expanding the availability of new targets for antibody drug conjugates
- Opening solid tumor opportunities for T-cell engaging bispecifics
- Harnessing the anti-cancer power of cytokines
- EpCAM (CX-2051)
- CD71 (CX-2029)
- EGFR-CD3
- Multiple programs in development
- IFN-a2b (CX-801)
- Multiple other programs in development